

Your golden opportunity to treat BRD and control its fever in just one dose.
When you spot BRD, choose RESFLOR GOLD to target the infection and quickly reduce fever.
The industry-standard dual therapy.
Florfenicol is the same powerful antibiotic used in NUFLOR® (florfenicol), a trusted brand for over 25 years.
It’s proven to fight all four major BRD pathogens:
- Mannhemia haemolytica
- Pasteurella multocida
- Histophilus somni
- Mycoplasma bovis
Flunixin meglumine is the same NSAID used in BANAMINE® (flunixin meglumine injection).
It’s proven to quickly reduce fever associated with BRD. Reducing fever is a critical component of treatment, as reducing fever is proven to support recovery.

Together, they produce consistent results, and you can see improvement in as little as six hours.3
Why RESFLOR GOLD is the smart way to target BRD.
Bovine respiratory disease can lead to permanent lung damage and lower production.1 That puts profitability at risk. By combining the antibiotic florfenicol with the non-steroidal anti-inflammatory (NSAID) flunixin meglumine, RESFLOR GOLD helps treat BRD and the fever that often accompanies it. By quickly reducing fever, RESFLOR GOLD helps provide visible relief and promotes rapid recovery.
Find RESFLOR GOLD online or at an animal health supplier near you.
See RESFLOR GOLD in action.
BRD is one of the most costly diseases in cattle production. To help limit losses, it’s important to choose a product that provides visible relief and promotes rapid recovery. RESFLOR GOLD does this in as little as six hours after just one dose. See for yourself.
Spotting the signs of BRD.2
The signs of BRD are usually seen 5-14 days after stressful events such as:
- Commingling
- Processing
- Weaning
- Changes to diet
- Changes in weather

As for the signs themselves, here’s what to keep an eye out for:
- Lack of appetite
- Droopy ears
- Rapid breathing
- Soft coughing
- Runny eyes
- Head hanging low
- Less interest in their environment
When you see signs in your herd, consider it your golden opportunity to knock it back with proven, powerful RESFLOR GOLD.
RESFLOR GOLD®
(Florfenicol and Flunixin Meglumine)
Product Description
RESFLOR GOLD® is an injectable solution of the synthetic antibiotic florfenicol and the non-steroidal anti-inflammatory drug (NSAID) flunixin. Each milliliter of sterile RESFLOR GOLD® contains 300 mg florfenicol, 16.5 mg flunixin as flunixin meglumine, 300 mg 2-pyrrolidone, 35 mg malic acid, and triacetin qs.

Treating both BRD and the fever that accompanies it is easy with the dual therapy of RESFLOR GOLD.
GIVEN SUBCUTANEOUSLY IN THE NECK.

ADMINISTERED AT A DOSE RATE OF 6 ML/100 LBS.

38-DAY WITHDRAWL PRIOR TO SLAUGHTER.

COMBINES TWO THERAPIES IN A SINGLE DOSE.
Indications
RESFLOR GOLD® is indicated for treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis, and control of BRD-associated pyrexia in beef and non-lactating dairy cattle.
For subcutaneous use in beef and non-lactating dairy cattle only.
Not for use in female dairy cattle 20 months of age or older or in calves to be processed for veal.
Diseases
- Bovine respiratory disease (BRD)
- Histophilus somni
- Mannheimia haemolytica
- Mycoplasma bovis
- Pasteurella multocida
Dosage
RESFLOR GOLD® should be administered once by subcutaneous injection at a dose rate of 40 mg florfenicol/kg body weight and 2.2 mg flunixin/kg body weight (6 mL/100 lb). Do not administer more than 10 mL at each site. The injection should be given only in the neck. Injection sites other than the neck have not been evaluated. For the 500 mL vial, do not puncture the stopper more than 20 times.
RESFLOR GOLD® Dosage Guide*
| ANIMAL WEIGHT (lbs) | DOSAGE (mL) |
|---|---|
| 100 | 6.0 |
| 200 | 12.0 |
| 300 | 18.0 |
| 400 | 24.0 |
| 500 | 30.0 |
| 600 | 36.0 |
| 700 | 42.0 |
| 800 | 48.0 |
| 900 | 54.0 |
| 1000 | 60.0 |
Recommended Injection Location
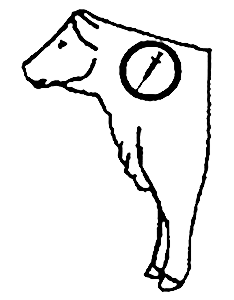
* Do not administer more than 10 mL at each site.
Contraindications
Do not use in animals that have shown hypersensitivity to florfenicol or flunixin.
Precautions
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Patients at greatest risk for adverse events are those that are dehydrated, on diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully monitored. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that have not been previously diagnosed. Since many NSAIDs possess the potential to produce gastrointestinal ulceration, concomitant use of RESFLOR GOLD® with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided or closely monitored.
Flunixin is a cyclo-oxygenase inhibitory NSAID, and as with others in this class, adverse effects may occur with its use. The most frequently reported adverse effects have been gastrointestinal signs. Events involving suspected renal, hematologic, neurologic, dermatologic, and hepatic effects have also been reported for other drugs in this class.
Not for use in animals intended for breeding purposes. The effects of florfenicol on bovine reproductive performance, pregnancy, and lactation have not been determined. Toxicity studies in dogs, rats, and mice have associated the use of florfenicol with testicular degeneration and atrophy. NSAIDs are known to have potential effects on both parturition and the estrous cycle. There may be a delay in the onset of estrus if flunixin is administered during the prostaglandin phase of the estrous cycle. The effects of flunixin on imminent parturition have not been evaluated in a controlled study. NSAIDs are known to have the potential to delay parturition through a tocolytic effect.
RESFLOR GOLD®, when administered as directed, may induce a transient reaction at the site of injection and underlying tissues that may result in trim loss of edible tissue at slaughter.
Adverse Reactions
Transient inappetence, diarrhea, decreased water consumption, and injection site swelling have been associated with the use of florfenicol in cattle. In addition, anaphylaxis and collapse have been reported post-approval with the use of another formulation of florfenicol in cattle. In cattle, rare instances of anaphylactic-like reactions, some of which have been fatal, have been reported, primarily following intravenous use of flunixin meglumine.
Supplied
100 mL sterile, multiple-dose, glass vials.
250 mL sterile, multiple-dose, glass vials.
500 mL sterile, multiple-dose, glass vials.
Storage
Do not store above 30°C (86°F). Use within 28 days of first use.
Warnings
NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. This product contains material that can be irritating to skin and eyes. Avoid direct contact with skin, eyes, and clothing. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. Consult a physician if irritation persists. Accidental injection of this product may cause local irritation. Consult a physician immediately. The Material Safety Data Sheet (MSDS) contains more detailed occupational safety information.
Important Safety Information
RESFLOR GOLD: Not for use in humans. Keep out of reach of children. Do not use in animals that have shown hypersensitivity to florfenicol or flunixin. Avoid direct contact with skin, eyes and clothing as product contains materials that can be irritating. Animals intended for human consumption must not be slaughtered within 38 days treatment. This product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. A withdrawal period has not been established in pre-ruminating calves. Do not use in calves to be processed for veal. Not for use in animals intended for breeding purposes. See package insert for complete information.
NUFLOR INJECTABLE SOLUTION: Do not use in animals that have shown hypersensitivity to florfenicol or animals intended for breeding purposes. Transient inappetence, diarrhea, decreased water consumption, injection site swelling, anaphylaxis and collapse have been associated with the use of florfenicol in cattle. Do not use in calves to be processed for veal or in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. Animals intended for human consumption must not be slaughtered within 28 days of the last intramuscular treatment or 38 days of subcutaneous treatment. A withdrawal period has not been established in pre-ruminating calves. Avoid direct contact with skin, eyes, and clothing. Pregnant women should wear gloves and exercise caution or avoid handling this product. For complete information, see the product package insert.
BANAMINE: Do not use BANAMINE (flunixin meglumine injection) within 48 hours of expected parturition. Do not use in animals showing hypersensitivity to flunixin meglumine. Approved only for intravenous administration. Intramuscular administration has resulted in violative residues in the edible tissues of cattle sent to slaughter. Cattle must not be slaughtered for human consumption within 4 days of the last treatment. Milk that has been taken during treatment and for 36 hours after the last treatment must not be used for food. Not for use in dry dairy cows. A withdrawal period has not been established for this product in preruminating calves. Do not use in calves to be processed for veal. For complete information, see accompanying product package insert.
Contact
U.S. only: Merck Animal Health livestocktechsrvc@merck-animal-health.com or call 1-800-211-3573
For more information regarding efficacy and safety data, go to productdata.aphis.usda.gov
For additional information, please see the product label.
References
1. Lekeux P. Bovine respiratory disease complex: a European perspective. Bov Pract. 1995;29:71-75.
2. Griffin D. Economic impact associated with respiratory disease in beef cattle. Vet Clin North Am Food Anim Pract. 1997;13:367–377.
3. See product label.

Get the latest updates! Sign up to receive cattle health management insights, industry news and more sent straight to your inbox.
